FREQUENTLY ASKED QUESTIONS
We answer to your questions
ABOUT QREM CYTOKINE TECHNOLOGY
What is Qrem Cytokine?
Qrem Cytokine is an innovative technology for obtaining cytokines which are involved in tissue regeneration. Based on patient’s own blood, Autologous Cytokine Rich Serum (ACRS) is obtained through a patented biological process that emulates an ex vivo tissular damage.
This product has the CE marking MDR00040 granted by Notified Body KIWA Cermet (nº 0476).
Is a Lab-in-a-Box technology, closed and automatic, that consists in a device (QREM DEVICE) and a single-use disposable sterile kit (KIT QREM CYTOKINE). It can be installed in all kind of doctor’s office, no matter how small it is. No specific training or special facilitiy infrastructures are required.
The QREM DEVICE and the KIT QREM CYTOKINE are medical devices class IIa that are commercializing in Europe since 2018.
| QREM DEVICE: Desktop equipment | KIT QREM CYTOKINE: single-use disposable sterile kit |
 |  |
What are the differences between ACRS and Platelet Rich Plasma (PRP)?
The main difference is the use of leukocytes during the ACRS process which allows to obtain anti-inflammatory cytokines.
ACRS does not contain platelets, fibrinogen nor other type of blood cells but it directly contains the anti-inflammatory and anabolic cytokines (also known as growth factors) which are involved in the tissular regeneration process. While PRP contains mainly the mediator (platelets), ACRS directly contains the active ingredient (cytokines); and, while PRP must be activated for the growth factors it contains to be released (either with calcium chloride or endogenously), ACRS is obtained through a natural process without additives or incubation that replicates endogenous signaling pathways.
How does Qrem Cytokine work?

Qrem Cytokine technology carries out a multi-step process which takes 36 minutes. This process emulates an ex vivo tissular damage that produces the blood cells to be activated and to release the regeneration modulating cytokines which are contained in ACRS.
To obtain the ACRS, the KIT QREM CYTOKINE is subjected to a cycle of 8 processing steps segmented into 3 phases with a total duration of 36 minutes. The first phase corresponds to cell fractionation, the second to the activation of platelets and white blood cells, and the third corresponds to the release of cytokines and growth factors. All steps are performed automatically by the QREM DEVICE.
What regulation applies to QREM DEVICE and KIT QREM CYTOKINE?
According to Annex VIII of Regulation (EU) 2017/745, QREM DEVICE and KIT QREM CYTOKINE are non-implantable Class IIa medical devices and are indicated for obtention of autologous platelet by-products used in all musculoskeletal or dermatological clinical specialty treatments that require the injection of platelet derivatives, such as growth factors, cytokines, and biological mediators to achieve the stimulation and healing acceleration of both soft tissue and hard tissue (including bone).
Who is Tecnologia Regenerativa Qrem S.L.?
Tecnologia Regenerativa Qrem S.L. is a Barcelona-based company founded in 2016 with medical product installation and fabrication license number 7096-PS granted by Spanish Agency of Medicines and Medical Devices (AEMPS).
ABOUT OSTEOARTHRITIS AND REGENERATIVE MEDICINE
What is Osteoarthritis (OA)?
Osteoarthritis (OA) is the commonest form of arthritis. A degenerative and chronic disorder associated with damage to the cartilage and surrounding tissues. Characterized by damage and loss of cartilage, it causes friction between bones, as a consequence huge pain and disability.
Joints of the knees, hips, back (spine), fingers, thumbs and neck are commonly affected.
The conventional therapies used in the initial phases are analgesics, anti-inflammatories and chondroprotective. When pain and dysfunction increase, injections of corticosteroids, hyaluronic acid and/or PRP (Platelet Rich Plasma) are prescribed. These treatments may have limitations in the doses that can be delivered and ultimately a prosthesis is implanted.
In Spain, between 60 and 100 new cases are diagnosed every year per 100,000 people. Worldwide, 9,6% of men and 18% of women aged>60 suffer from OA.
|
OA is characterized by an unbalance of cytokines that produce cartilage degradation (pro-inflammatory cytokines) and cytokines that try to heal it (anti-inflammatory and growth factors). Cartilage degradation is produced by inflammatory activation carried out by some cytokines such as IL-1β, IL-6, IL-2, IL-8 y TNF among others. While healing is carried out by cytokines IGF-1, BMP, PDGF-BB, TGF-β, IL-4, IL-10 y IL-13. |
How does regenerative medicine contribute?
Regenerative medicine is the name received by the therapies that are applied to tissue regeneration and organism structure improvement.
The goal of regenerative medicine is to repair damaged tissues by means of cells, proteins and/or biomaterials by addressing directly to the disease root cause, not only to symptoms.
Regenerative therapies based on stem cells have proven to be very promising in the treatment of degenerative diseases related to age. However, more studies are required to widen clinic effectiveness.
Recently, a step forward has been taken in use of immune system modulating elements, like cytokines or exosomes. These elements support existing regenerative strategies. As an example: mesenchyme cells of bone marrow or adipose tissue, and in some cases they can substitute them.
Regenerative therapies will be the next medicine revolution.
What are cytokines?
Cytokines are proteins released by blood cells and platelets. These proteins are messages used by cells to manage different processes.
There are several families of cytokines, including proinflammatory cytokines, which promote inflammation; anti-inflammatory cytokines, which have the opposite effect, and growth factors*, which stimulate cell proliferation.
Our body works properly when pro-inflammatory, anti-inflammantory and growth factors are balanced, in other words, when homeostasis exists. Even though inflammation is a necessary process of regeneration, this state cannot be prolonged for an extended period of time since tissues can be damaged permanently and this would cause cartilage degradation.
*Growth factors: It is a type of cytokine mainly related to cell proliferation and growth. These cytokines are mainly stored in blood platelets. |
Available treatments supported by Spanish public health system
Nowadays, Spanish public health system does not offer an effective treatment treatment for musculoskeletal pathologies before requiring surgery. Analgesics (Paracetamol, Acetaminophen,…), NSAIDs (Ibuprofen, Naproxen, Indometacin,…), Opioids (Tramadol,…) are the most commont drugs used for pain reduction. These drugs must be taken under medical prescription.
Intra-articular infiltrations* of corticosteroids are also usually prescribed in cases of increased pain and inflammation.
At some medical centers, hyaluronic acid infiltrations could be carried out.
However, the aforementioned treatments aim to reduce pain, inflammation and increase the quality of life of the patient while waiting for surgery.
*Intra-articular infiltration: Is an ambulatory treatment that consist in an injection in the articulation and which does not require anesthesia. *Hyaluronic acid: it acts as a lubricant in the articulation which partially reduces pain. |
Treatments not included in Spanish health system
Private health services offer regenerative medicine treatments oriented to slow down and even stop the progression of musculoskeletal pathologies before requiring surgery.
As these treatments will give more evidences of their efficacy, it is foreseeable that they will be included into Spanish public health system.
Regenerative medicine treatments
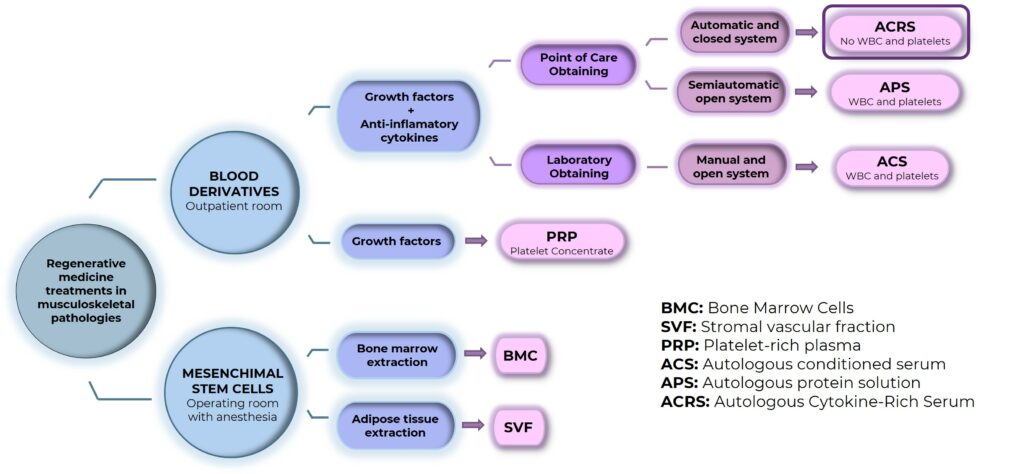
In Spain, Regenerative medicine treatments can be divided into two different groups:
- Mesenchymal stem cells* treatments obtained from bone marrow or adipose tissue. This kind of treatment require surgery and anesthesia.
In some cases, especially when treating with bone marrow samples, a cell culture is carried out in laboratories, and it can take several days. In these cases, it is considered as an advanced therapy and an authorization from sanitary regulators is required to guarantee a correct culture (facilities, process, transport, preservation, etc.) as well as a correct clinical use.
| *Meshenchymal stem cells: they are multipotencial cells that can be differenciated (with appropiate signals) into different cell types such as skeletal tissues, cartilage, bones or fat. |
- Treatments with non-substitute products obtained from the patient’s own venous blood. These kind of treatments are outpatients. For obtaining the product, the medical center must have closed devices or, in case they are opened, it is necessary to have the appropriated equipment in order to avoid possible contaminations. In this category, following treatments can be found.
- Plateled Rich Plasma and/or growth factors*(PRP)
- Autologous* Condicioned Serum (ACS).
- Autologous Protein Solution (APS).
- Autologous Cytokine Rich Serum (ACRS) obtained by KIT QREM CYTOKINE and QREM DEVICE.
PRP, ACS, APS and ACRS are not cell therapies or treatment nor advanced medicine therapies. However, they must be administrated always under medical presctription and guarantee some quality requirements of production.
*Growth factors: It is a type of cytokine mainly related to cell proliferation and growth. These cytokines are mainly stored in blood platelets. *Autologous: from the patient |
What is PRP?
PRP is the acronym of Platelet Rich Plasma. PRP is a blood-derived product obtained through blood centrifugation and it contains a high concentration of platelets (between 4 to 6 times normal values). When platelets are activated, they release growth factors that contribute to stimulate the tissue regeneratiom.
Platelets can be activated naturally if they come into contact with the body’s own activation triggers, or artificially by adding calcium chloride before infiltration.
What is ACS?
ACS is the acronym of Autologous Conditioned Serum. ACS is a blood-derived product obtained by an incubation process of blood of 6 hours or more at a temperature of 37ºC. With this incubation, anti-inflammatory cytokines from leukocytes are released.
What is APS?
APS is the acronym of Autologous Protein Solution. APS contains high concentration of leukocytes and platelets. The process does not include incubation but it requires blood processing with an opened system. Part of leukocytes and platelets are activated during this process releasing anti-inflammatory cytokines and growth factors. The rest can be activated after injection when contacting with coagulation factors produced by the body.
What is ACRS?
ACRS is the acronym of Autologous Cytokine Rich Serum. It is a cell-free serum with a balanced concentration of cytokines from plasma, leukocytes and platelets. The ACRS directly contains the active ingredient, cytokines and growth factors. The process of obtaining the ACRS is carried out using the KIT QREM CYTOKINE and the QREM DEVICE device (with Lab-in-a-box* technology) and is indicated for all treatments in musculoskeletal or dermatological clinical specialties that require platelet byproducts, such as growth factors, cytokines and biological mediators to achieve the stimulation and acceleration of the repair of both soft and hard tissues (including bone). Autologous platelet byproducts can be used for the treatment of:
- Osteoarthritis
- Tendinopathy (lateral epicondylitis, carpal tunnel syndrome, rotator cuff tears, plantar fasciitis, Achilles tendinopathy, and patellar tendinopathy)
- Musculoskeletal soft tissue injuries
- Healing of bone fractures
- Chronic low back pain
- Correction of hypertrophic and atrophic scars
- Chronic wounds that do not heal.
*Lab-in-a-box: Totally automatic technology, closed and sterile which enables to be used as an outpatient treatment with no risk. QREM CYTOKINE system for obteining ARCS is a Lab-in-a-box system. |